BD Integrated Diagnostic Solutions
Help advance care for patients throughout their diagnostic journey, while also addressing your testing needs

Your diagnostic challenges are interconnected. Your solution should be too.
98% of hospital inpatients undergo some form of diagnostic testing.1 Diagnostic tests for blood culture, blood chemistry and urine affect nearly every patient in your facility. These tests are critical to determining the next step in their treatment. And while ensuring patient care is your primary priority, you may also face other challenges such as complex tests, suboptimal workflows, preanalytical errors and staffing shortages.
These concerns are not siloed – they impact each other, and your patients. What if you could integrate your facility’s diagnostic pathway, with solutions at each step working together? Watch the video and explore the patient journey below to see how BD can help:
Enabling you to advance patient care at each step
From collecting specimens to reporting results, the diagnostic pathway has multiple points, each with the potential to optimize efficiencies and advance the quality of patient care. BD offers a comprehensive portfolio of integrated solutions designed to help your facility elevate the patient experience and position your lab as a vital resource, rather than a necessary cost center.

The BD Veritor™ Plus System offers convenient point-of-care testing for SARS-CoV-2*, Influenza A+B, Group A Streptococcus, and respiratory syncytial virus and combines rapid, reliable results with ease of implementation and simple workflow options.
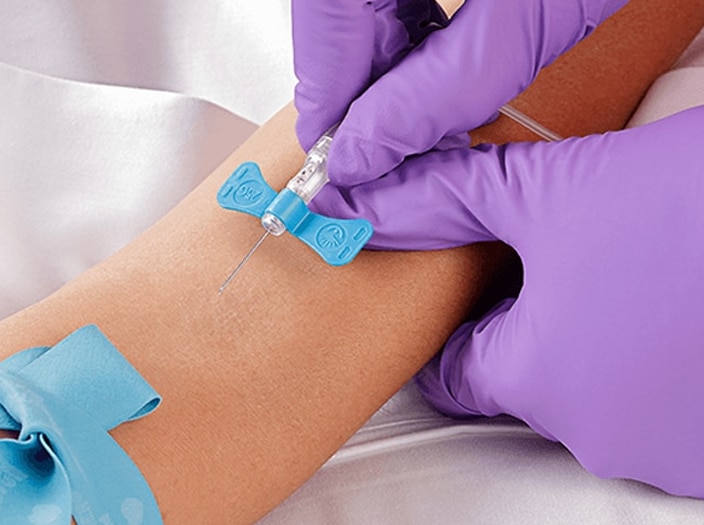
The BD Vacutainer® product family enhances sample quality while helping protect nurses, phlebotomists and other caregivers from costly accidental needlestick injuries. The brand also helps reduce retests, recollects and instrument downtime to improve lab productivity and workflow.
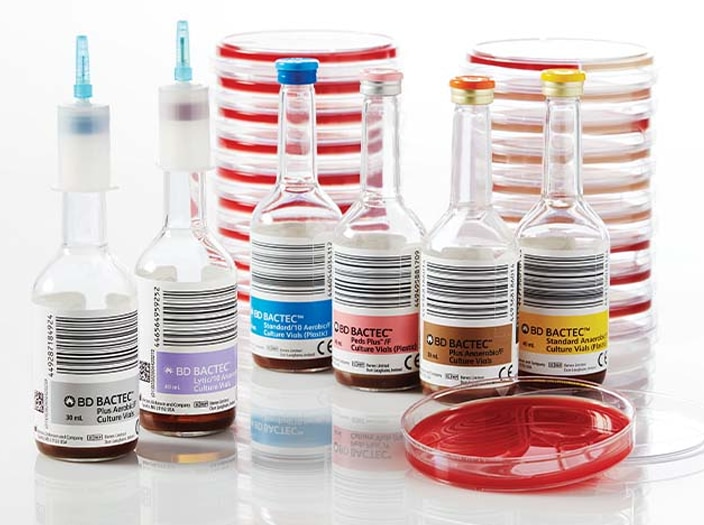
Decrease time to detection during the analytical phase with a full spectrum of blood culture media solutions. A full line of blood culture media developed specifically for the detection of aerobes, anaerobes, yeast, fungi and mycobacteria to help improve time to detect and organism recovery2 from both adult and pediatric patients.

We believe in innovative medical technology and the potential of the future. We’ve developed a women’s health portfolio with innovative instruments and assays to aid in the diagnosis of cervical cancer, sexually transmitted infections, vaginitis, and antepartum screening. Solutions designed to deliver the accurate results you need to keep women healthy and strong.
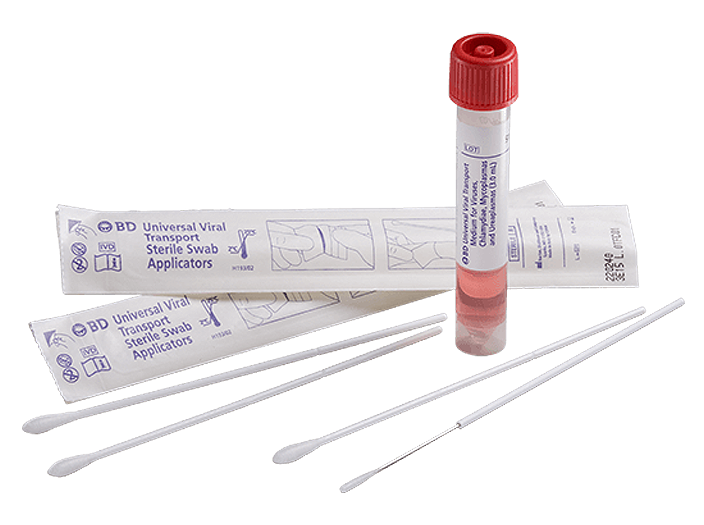
The BD universal viral transport (UVT) system is designed to transport viruses, chlamydiae, mycoplasmas and urea plasmas at room temperature. It is one of the only systems that may be stored and transported at 2°C to 25°C—all in one formulation.
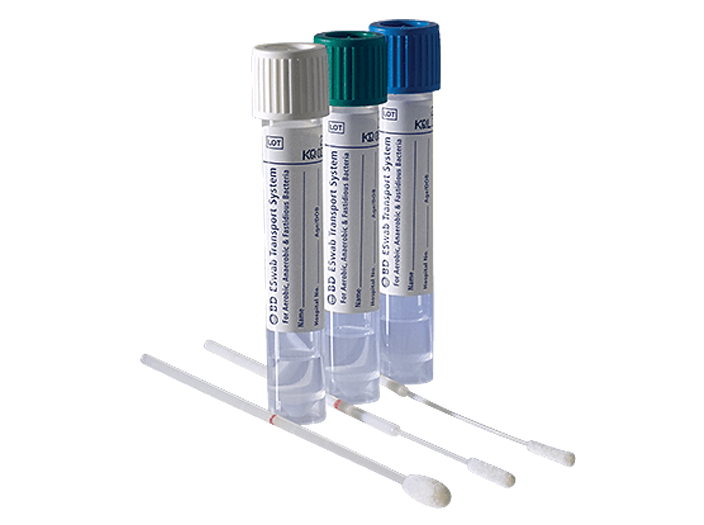
The system helps collect clinical specimens containing aerobic, anaerobic and fastidious bacteria from the collection site, and transport them to the testing laboratory. In the lab, BD ESwab™ specimens are processed using standard clinical lab operating procedures for bacterial culture. The unique design of the flocked swab ensures optimal elution of the specimen into the transport medium.

BD Kiestra™ laboratory automation solutions are uniquely positioned to offer standardized and scalable solutions for inoculation, incubation, plate imaging and reading and follow-up testing.
BD Kiestra™ solutions help prepare laboratories to meet these changing demands and achieve efficient, timely and cost-effective clinical bacteriological testing.
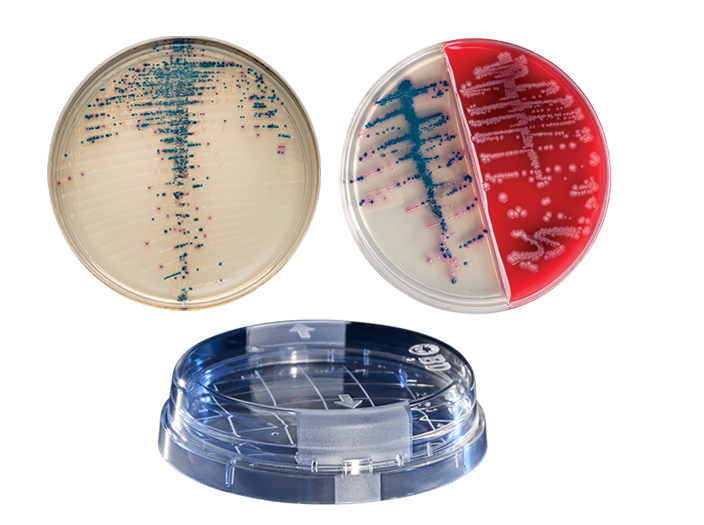
BD BBL™ prepared plated media enable the isolation of microorganisms from samples or specimens. Ready for immediate use, they are routinely available in several types of Petri-style dishes, containing a variety of media.

It has advanced fluorescence detection technology, exceptional media performance and instrument reliability—adding vial-activated workflow, advanced ergonomics, blood culture observation and customer-focused data management. The easy-to-use workflow comes in a compact, modular design, which can be adapted to multiple space- and time-saving configurations for your laboratory staff.

The BD MAX™ system offers you an efficient path to improved clinical outcomes by combining and automating extraction and thermocycling into a single platform capable of running both FDA-cleared and open system assays. Flexibility and standardization allow you to address the breadth of your testing needs.

Delivering efficiency, flexibility, and performance to meet your laboratories' evolving molecular diagnostic needs. The BD COR™ System uses multiplex PCR assay design and requires less than 1 hour of daily hands-on time for the full BD COR™ system workflow and only 2 interactions.

The BD Phoenix™ M50 instrument provides clinicians with accurate and timely identification and susceptibility results to help guide their therapy and patient management decisions. BD Phoenix™ panels provide accurate, rapid identification and susceptibility results for most clinically significant aerobic and facultative anaerobic Gram-negative and Gram-positive bacteria, as well as identification of yeast and yeast-like organisms.

Take an integrated approach to BSI testing. A bloodstream infection (BSI) can lead to a dysregulated immune response if untreated. BSI’s can lead to potentially life-threatening conditions, including sepsis. To make substantial improvements to BSI testing, consider the entire diagnostic pathway – from blood culture collection to result.

BD Synapsys™ solution enables laboratories to address challenges and improve laboratory outcomes. It enables labs to maximize performance using enhanced microbiology diagnostic informatics through a single, advanced platform with an intuitive, personalized user interface.
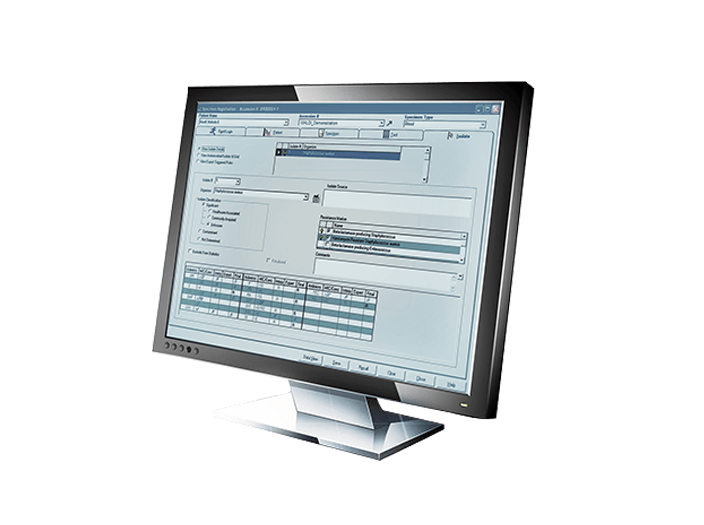
The BD EpiCenter™ microbiology data management system provides advanced data management for BD microbiology systems. It helps integrate these multiple platforms, facilitate improved workflow, analyze data and generate reports, including those used for epidemiological tracking.
5 reason to partner with BD on Integrated Diagnostic Solutions
to understand your current challenges and unmet needs aimed at revealing the most effective ways to address clinical quality & workflow impact using BD solutions
to help you identify and fill practice gaps by using BD solutions
to increase proficiency and minimize the learning curve
to address your queries and testing needs
to streamline testing workflows at your facility
Our comprehensive portfolio is backed by our leading-edge science, innovation technology, proven expertise and support services, enabling you to address your ongoing clinical and operational needs.
Let’s advance diagnostic value and impact in your facility.
Get in touch with a sales representative
Diagnostic tests classify infections and guide therapies, enabling clinicians to implement effective antimicrobial interventions
-

BD Integrated Diagnostic Solutions aim to optimize patient care pathways through essential tests crucial for making treatment decisions.
-

BD Integrated Diagnostic Solutions are designed to support your ability to minimize the effort needed to get accurate diagnostic results and help keep your lab ahead of the curve.
-
BD aims to equip pharmacists with integrated point-of-care diagnostic solutions, enhancing convenience and diagnostic confidence.
-
BD diagnostic solutions optimize patient care pathways through essential tests crucial for treatment decisions.
References
References:
- Ngo A, Gandhi P, Miller WG. Frequency that laboratory tests influence medical decisions. J ApplLab Med. 2017;1(4):410-414.
- Hawkins R. Managing the pre- and post-analytical phases of the total testing process. Ann Lab Med. 2012;32:5-16.
Notes:
* The BD Veritor™ System for Rapid Detection of SARS-CoV-2 is intended for the qualitative detection of SARS-CoV-2 nucleocapsid antigens in direct anterior nasal swabs from individuals who are either suspected of COVID-19 by their health care provider within the first five days of the onset of symptoms, or from individuals without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two or three days with at least 24 hours and no more than 48 hours between tests. This product has not been FDA cleared or approved; but has been authorized by FDA under an EUA for use by authorized laboratories. RSV=respiratory syncytial virus; SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. This product has been authorized only for the detection of proteins from SARS-CoV-2, not for any other viruses or pathogens. This product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated, or authorization is revoked sooner. Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C §263a, that meet the requirements to perform moderate, high or waived complexity tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
